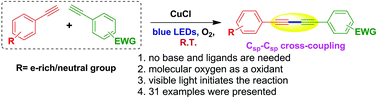
A novel visible-light-promoted copper-catalysed process for the Csp–Csp cross-coupling reaction of terminal alkynes at room temperature is described. The current photochemical method is simple, highly functional group compatible, and more viable towards the construction of bio-active 1,3-unsymmetrical conjugated diynes without the need of bases/ligands, additives and expensive palladium/gold catalysts.
Visible-light-activated copper(I) catalyzed oxidative Csp-Csp cross-coupling reaction: efficient synthesis of unsymmetrical conjugated diynes without ligands and base
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC01463A, Communication
DOI: 10.1039/C6GC01463A, Communication
Arunachalam Sagadevan, Ping-Chiang Lyu, Kuo Chu Hwang
An efficient and eco-friendly approach to Csp-Csp cross-coupling of terminal alkynes for the construction of unsymmetrical conjugated diynes via a visible-light-induced CuCl catalysed process at room temperature is described.
An efficient and eco-friendly approach to Csp-Csp cross-coupling of terminal alkynes for the construction of unsymmetrical conjugated diynes via a visible-light-induced CuCl catalysed process at room temperature is described.
Communication
Visible-light-activated copper(I) catalyzed oxidative Csp–Csp cross-coupling reaction: efficient synthesis of unsymmetrical conjugated diynes without ligands and base
View original post 70 more words