[76] Horner-Wadsworth-Emmons (HWE) Olefination 1958
Aryl-Aryl Coupling
A collection of various aryl-aryl homo and cross-coupling reactions, in particular featuring Pd-catalyzed major named cross coupling transformations: Kumada, Negishi, Stille, Suzuki, and Hiyama.
NEW DRUG APPROVALS HITS 20 LAKH VIEWS IN 218 COUNTRIES — All About Drugs
NEW DRUG APPROVALS https://newdrugapprovals.org/ ALL ABOUT DRUGS, LIVE, BY DR ANTHONY MELVIN CRASTO, WORLDDRUGTRACKER, HELPING MILLIONS, 9 MILLION HITS ON GOOGLE, PUSHING BOUNDARIES,2.5 LAKH PLUS CONNECTIONS WORLDWIDE, 18 LAKH PLUS VIEWS ON THIS BLOG IN 216 COUNTRIES, THE VIEWS EXPRESSED ARE MY PERSONAL AND IN NO-WAY SUGGEST THE VIEWS OF THE PROFESSIONAL BODY OR THE…
via NEW DRUG APPROVALS HITS 20 LAKH VIEWS IN 218 COUNTRIES — All About Drugs
[86] Prilezhaev Epoxidation 1909 — ChemInfoGraphic
[89] Passerini 3-CR 1921 — ChemInfoGraphic
ANTHONY M CRASTO GETS PHARMA EXCELLENCE AWARD AT THE GOLDEN GLOBE TIGER AWARDS 2018
Pic…. Shobha crasto, Lionel and Aishal collecting my award named The Golden Globe Tigers Awards 2018, for Excellence in Pharma, at kuala lumpur, Malaysia, 23 april 2018 at Pullman city centre hotel, Kuala lumpur, Malaysia. Dr Anthony could not travel as he is wheelchair bound and 90 percent paralysed

DR ANTHONY M CRASTO
The Golden Globe Tigers Award 2018 is the highest recognition amongst individual and organizations who have achieved the highest levels of standards and benchmark in numerous areas such as CSR, Pharma, Social Media & Digital Marketing, Education Leadership Award and so on. The award ceremony took place in Pullman Kuala Lumpur City Centre Hotel & Residences on the 23rd of April, where a number of respectable attendees were present not only from Malaysia but from many different parts of the world such as Iceland, Saudi Arabia, India, China, South Africa and such!
The Golden Globe Tigers Award not only…
View original post 659 more words
1,2 Diaminocyclohexane from Synthesis with Catalysts Pvt Ltd — All About Drugs
+91- 999-997-2051 info@synthesiswithcatalysts.com Website http://www.synthesiswithcatalysts.com/ bpk@synthesiswithcatalysts.com Axay Parmar Founder at Synthesis with Catalysts Pvt. Ltd, axayrp@gmail.com Basu Agarwal and Dr Razi Abdi shr@synthesiswithcatalysts.com, ba@synthesiswithcatalysts.com Synthesis with Catalysts Pvt Ltd was founded with an aim to help aromatic chemical, essential oil, pharmaceutical, API manufacturers to develop new products, increase productivity and improve production methodologies. We have an…
via 1,2 Diaminocyclohexane from Synthesis with Catalysts Pvt Ltd — All About Drugs
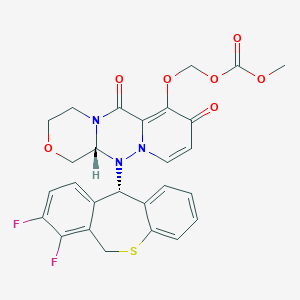
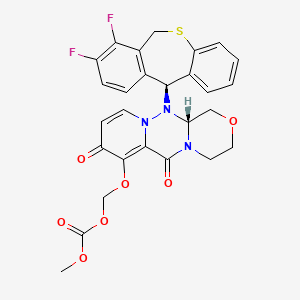
Baloxavir marboxil, バロキサビルマルボキシル , балоксавир марбоксил , بالوكسافير ماربوكسيل , 玛巴洛沙韦 ,
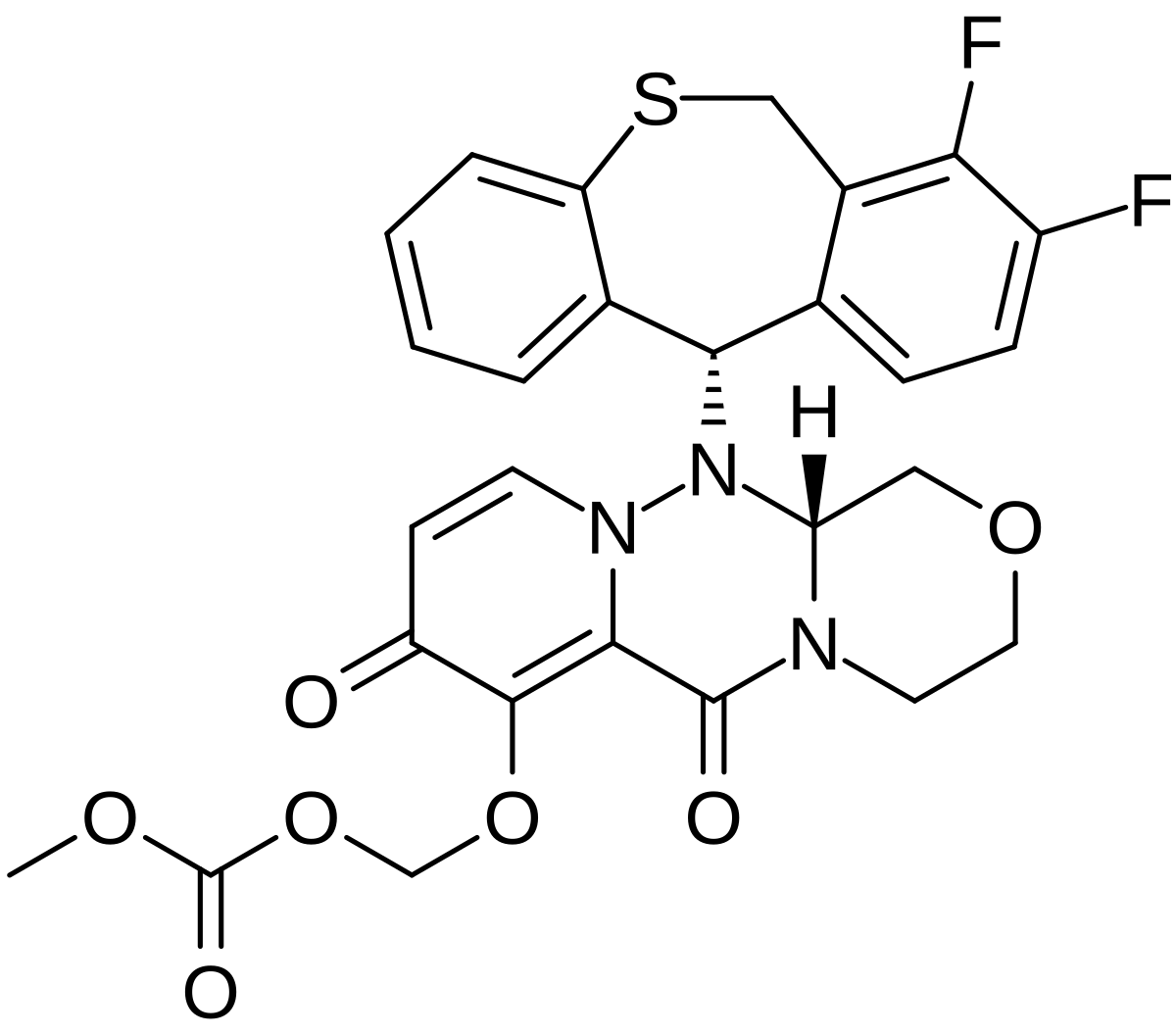
Baloxavir marboxil
バロキサビルマルボキシル
балоксавир марбоксил [Russian] [INN]
Carbonic acid, [[(12aR)-12-[(11S)-7,8-difluoro-6,11-dihydrodibenzo[b,e]thiepin-11-yl]-3,4,6,8,12,12a-hexahydro-6,8-dioxo-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazin-7-yl]oxy]methyl methyl ester
({(12aR)-12-[(11S)-7,8-Difluoro-6,11-dihydrodibenzo[b,e]thiepin-11-yl]-6,8-dioxo-3,4,6,8,12,12a-hexahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazin-7-yl}oxy)methyl methyl carbonate
- (((12aR)-12-((11S)-7,8-Difluoro-6,11-dihydrodibenzo(b,E)thiepin-11-yl)-6,8-dioxo-3,4,6,8,12,12ahexahydro-1H-(1,4)oxazino(3,4-C)pyrido(2,1-F)(1,2,4)triazin-7-yl)oxy)methyl methyl carbonate
- Carbonic acid, (((12aR)-12-((11S)-7,8-difluoro-6,11-dihydrodibenzo(b,E)thiepin-11-yl)-3,4,6,8,12,12a-hexahydro-6,8-dioxo-1H-(1,4)oxazino(3,4-C)pyrido(2,1-F)(1,2,4)triazin-7-yl)oxy)methyl methyl ester
Antiviral
In Japan the product is indicated for treatment influenza types A and B in adults and children
RG-6152
- Originator Shionogi
- Developer Roche; Shionogi
- Class Antivirals; Dibenzothiepins; Esters; Pyridines; Small molecules; Triazines
- Mechanism of Action Endonuclease inhibitors
Highest Development Phases
- Marketed Influenza A virus infections; Influenza B virus infections
- Phase III Influenza virus infections
- Preclinical Influenza A virus H5N1 subtype
Xofluza (TN)
Antiviral
| |
| Formula | C27H23F2N3O7S |
|---|---|
| Cas | 1985606-14-1 |
| Mol weight | 571.5492 |
| 2018/2/23 | PMDA | JAPAN | APPROVED | Baloxavir marboxil | Xofluza | Shionogi |
| バロキサビル マルボキシル Baloxavir Marboxil  C27H23F2N3O7S : 571.55 [1985606-14-1] |


https://chem.nlm.nih.gov/chemidplus/sid/1985606141
Baloxavir marboxil (trade name Xofluza…
View original post 2,566 more words
ELECLAZINE, элеклазин , إيليكلازين , 依来克秦 , REVISITED
ELECLAZINE
GS-6615
| Molecular Formula: | C21H16F3N3O3 |
|---|---|
| Molecular Weight: | 415.372 g/mol |
1443211-72-0
4-(pyrimidin-2-ylmethyl)-7-[4-(trifluoromethoxy)phenyl]-2,3-dihydro-1,4-benzoxazepin-5-one
4-(pyrimidin-2-ylmethyl)-7-[4-(trifluoromethoxy)phenyl]-2,3,4,5- tetrahydro-1,4- benzoxazepin-5-one
7-(4-(Trifluoromethoxy)phenyl)-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-one
1,4-Benzoxazepin-5(2H)-one, 3,4-dihydro-4-(2-pyrimidinylmethyl)-7-[4-(trifluoromethoxy)phenyl]-
Eleclazine; UNII-PUY08529FK; 1443211-72-0; GS-6615; PUY08529FK; 4-(pyrimidin-2-ylmethyl)-7-(4-(trifluoromethoxy)phenyl)-3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-on
- Phase III Long QT syndrome
| INGREDIENT | UNII | CAS |
|---|---|---|
| Eleclazine Hydrochloride | 4R1JP3Q4HI | 1448754-43-5 |
Eleclazine has been used in trials studying the treatment of LQT2 Syndrome, Long QT Syndrome, Ischemic Heart Disease, Ventricular Arrhythmia, and Long QT Syndrome Type 3, among others.
In 2015, orphan drug designation was assigned to the product by the FDA for the treatment of congenital long QT syndrome.

- Originator Gilead Sciences
- Class Antiarrhythmics; Ischaemic heart disorder therapies; Pyrimidines; Small molecules; Vasodilators
- Mechanism of Action Sodium channel antagonists
Highest Development Phases
- Phase III Long QT syndrome
- Phase II/III Hypertrophic cardiomyopathy
- Phase II Ventricular arrhythmias
- No development reported
View original post 2,322 more words
Fosravuconazole L-lysine ethanolate, ホスラブコナゾール L-リシンエタノール付加物

C23H2F2N5O5PS C6H14N2O2
C6H14N2O2 C2H6O : 739.73
C2H6O : 739.73
[914361-45-8]
[(2R,3R)-3-[4-(4-cyanophenyl)-1,3-thiazol-2-yl]-2-(2,4-difluorophenyl)-1-(1,2,4-triazol-1-yl)butan-2-yl]oxymethyl dihydrogen phosphate;(2S)-2,6-diaminohexanoic acid;ethanol
L-Lysine [[(2R,3R)-3-[4-(4-cyanophenyl)-1,3-thiazol-2-yl]-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)butan-2-yl]oxy]methyl dihydrogen phosphate ethanol
BFE-1224
BMS-379224
E-1224
ravuconazole prodrugs, ravuconazole methyl phosphate
fosravuconazole bis(L-lysine)
ホスラブコナゾール L-リシンエタノール付加物
| Formula | C23H20F2N5O5PS. C6H14N2O2. C2H6O |
|---|---|
| CAS | 914361-45-8 |
| Mol weight | 739.727 |
Antifungal, Ergosterol biosynthesis inhibitor
Fungal infection; Onychomycosis; Trypanosoma cruzi infection
PMDA JAPAN APPROVED
| 2018/1/19 | PMDA | APPROVED | Fosravuconazole L-lysine ethanolate | Nailin | Sato Pharmaceutical
FOR Tinea, nail (onychomycosis) |
NOTE THIS STR
- 4-[2-[(1R,2R)-2-(2,4-Difluorophenyl)-1-methyl-2-[(phosphonooxy)methoxy]-3-(1H-1,2,4-triazol-1-yl)propyl]-4-thiazolyl]benzonitrile
- E 1224
- Fosravuconazole
- CAS 351227-64-0
Drugs for Neglected Diseases initiative (DNDi), under license from Eisai, is developing fosravuconazole for CD and eumycetoma
In February 2013, the drug was in phase II/III development by Seren Pharmaceuticals for onychomycosis in North America, Europe and Asia, including Japan,
In 2010, the product was licensed exclusively to Brain Factory (now Seren Pharma) for development, commercialization and sublicense in Japan for the…
View original post 719 more words
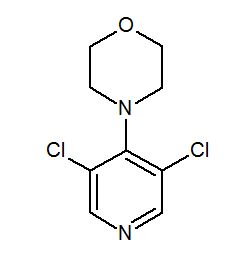
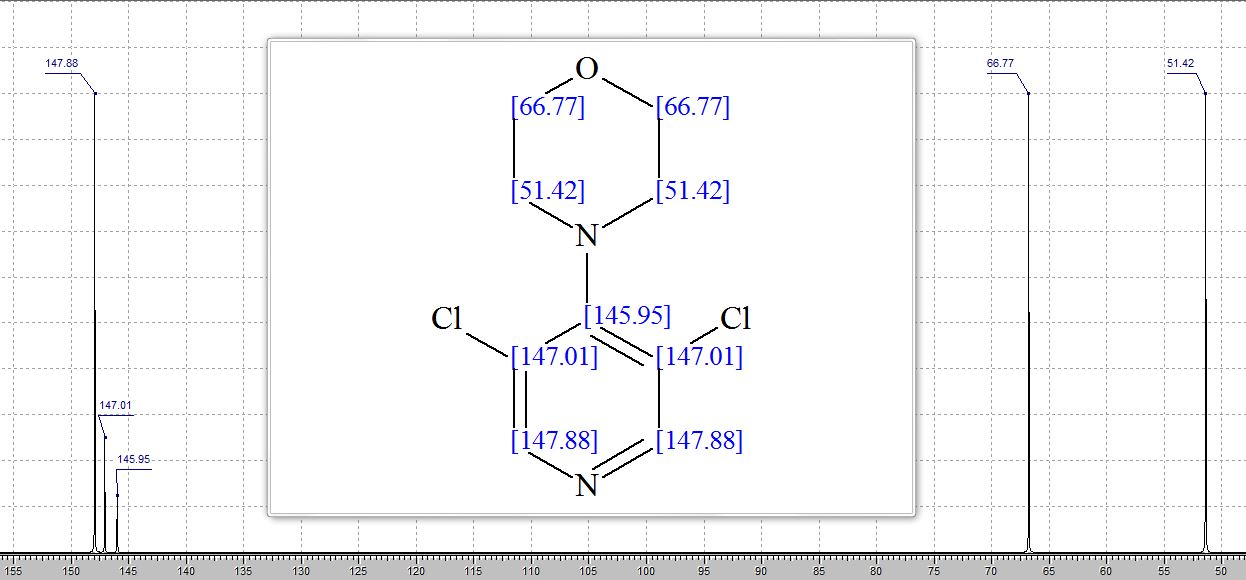
BRUSH UP YOUR NMR, 4-(3,5-Dichloropyridin-4-yl)morpholine
Preparation of 4-(3,5-Dichloropyridin-4-yl)morpholine (5).(Pichowicz, M.; Crumpler, S.; McDonald, E.; Blagg, J. Tetrahedron 2010, 66, 2398, DOI: 10.1016/j.tet.2010.01.101)


/////////////
Clc2cncc(Cl)c2N1CCOCC1
4,4,5,5-Tetramethyl-2-phenethyl-1,3,2-dioxaborolane — ORGANIC SPECTROSCOPY INTERNATIONAL
4,4,5,5-tetramethyl-2-phenethyl-1,3,2-dioxaborolanehttp://orgsyn.org/demo.aspx?prep=v94p0234 4,4,5,5-Tetramethyl-2-phenethyl-1,3,2-dioxaborolane (1) has the following physical and spectroscopic properties: Rf = 0.47 (3:97, ethyl acetate:pentane), the checkers report the following values for 1: Rf = 0.09 (3:97 ethyl acetate:pentane); Rf = 0.52 (10% EtOAc in hexanes); Merck silica gel 60 F254 plate; mp 38-39 °C; 1H NMR pdf(CDCl3, 400 MHz) δ: 1.18 (t, J = 8.4 Hz, 2H), 1.26 (s, 12H), 2.79 (t, J = 8.0 Hz, 2H), 7.16-7.22 (m,…
via 4,4,5,5-Tetramethyl-2-phenethyl-1,3,2-dioxaborolane — ORGANIC SPECTROSCOPY INTERNATIONAL
Pidotimod, 匹多莫德 , пидотимод , بيدوتيمود ,

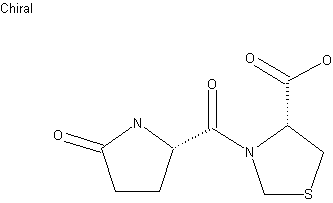
Pidotimod
H-Pyr-Thz-OH
(4R)-3-[(2S)-5-oxopyrrolidine-2-carbonyl]-1,3-thiazolidine-4-carboxylic acid
CAS 121808-62-6
Thymodolic acid, Pidotimod, Timodolic acid, PGT/1A, Axil, Onaka, Pigitil, Polimod
(4R)-3-(5-oxo-L-prolyl)-l ,3-thiazolidine-4-carboxylic acid, ITI 231723.
3-(L-pyroglutamyl)-L-thiazolidine-4-carboxylic acid
- 4-Thiazolidinecarboxylic acid, 3-[(5-oxo-2-pyrrolidinyl)carbonyl]-, [R-(R*,S*)]-
- (4R)-3-[[(2S)-5-Oxo-2-pyrrolidinyl]carbonyl]-4-thiazolidinecarboxylic acid
- Adimod
- Axil (pharmaceutical)
- Pigitil
| Pidotimod; 121808-62-6; (R)-3-((S)-5-Oxopyrrolidine-2-carbonyl)thiazolidine-4-carboxylic acid; Pidotomod; PGT/1A; Pidotimod [INN]; | |
| Molecular Formula: | C9H12N2O4S |
|---|---|
| Molecular Weight: | 244.26758 g/mol |
Stefano Poli, Corona Lucio Del
Pidotimod is an immunostimulant.[1]
Pidotimod, whose chemical name is (4R)-3-(5-oxo-L-prolyl)-l ,3-thiazolidine-4-carboxylic acid, was first disclosed in ITI 231723. It is a synthetic peptide-like molecule provided with an in vitro and in vivo immunomodulating action (Giagulli et al., International Immunopharmacology, 9, 2009, 1366-1373). The immune system assists in maintaining a homeostatic balance between the human body and all foreign substances. An abnormality in this balance may cause a defective or aberrant response towards non-self substances…
View original post 1,896 more words
(1) Lawesson’s reagent 1956 — ChemInfoGraphic
National award to Anthony Melvin Crasto for contribution to Pharma society from Times Network for Excellence in HEALTHCARE) | 5th July, 2018 | Taj Lands End, Mumbai, India
DR ANTHONY MEVIN CRASTO Conferred prestigious individual national award at function for contribution to Pharma society from Times Network, National Awards for Marketing Excellence ( For Excellence in HEALTHCARE) | 5th July, 2018 | Taj Lands End, Mumbai India
////////////National award, contribution to Pharma society, Times Network, Excellence in HEALTHCARE, 5th July, 2018, Taj Lands End, Mumbai, India, ANTHONY CRASTO
#hotpersoninawheelchair
#worlddrugtracker
National award to Anthony Melvin Crasto for contribution to Pharma society from Times Network for Excellence in HEALTHCARE) | 5th July, 2018 | Taj Lands End, Mumbai, India
DR ANTHONY MEVIN CRASTO Conferred prestigious individual national award at function for contribution to Pharma society from Times Network, National Awards for Marketing Excellence ( For Excellence in HEALTHCARE) | 5th July, 2018 | Taj Lands End, Mumbai India
////////////National award, contribution to Pharma society, Times Network, Excellence in HEALTHCARE, 5th July, 2018, Taj Lands End, Mumbai, India, ANTHONY CRASTO
#hotpersoninawheelchair
#worlddrugtracker
Development of an SNAr Reaction: A Practical and Scalable Strategy To Sequester and Remove HF

A simple and operationally practical method to sequester and remove fluoride generated through the SNAr reaction between amines and aryl fluorides is reported. Calcium propionate acts as an inexpensive and environmentally benign in situ scrubber of the hydrofluoric acid byproduct, which is simply precipitated and filtered from the reaction mixture during standard aqueous workup. The method has been tested from 10 to 100 g scale of operation, showing >99.5% decrease in fluoride content in each case. Full mass recovery of calcium fluoride is demonstrated at both scales, proving this to be a general, efficient, and robust method of fluoride abstraction to help prevent corrosion of glass-lined reactors.
Development of an SNAr Reaction: A Practical and Scalable Strategy To Sequester and Remove HF
///////////////aryl amines, calcium fluoride, fluoride sequestration, scale-up, SNAr reaction,
Development of an SNAr Reaction: A Practical and Scalable Strategy To Sequester and Remove HF

A simple and operationally practical method to sequester and remove fluoride generated through the SNAr reaction between amines and aryl fluorides is reported. Calcium propionate acts as an inexpensive and environmentally benign in situ scrubber of the hydrofluoric acid byproduct, which is simply precipitated and filtered from the reaction mixture during standard aqueous workup. The method has been tested from 10 to 100 g scale of operation, showing >99.5% decrease in fluoride content in each case. Full mass recovery of calcium fluoride is demonstrated at both scales, proving this to be a general, efficient, and robust method of fluoride abstraction to help prevent corrosion of glass-lined reactors.
Development of an SNAr Reaction: A Practical and Scalable Strategy To Sequester and Remove HF
View original post 122 more words
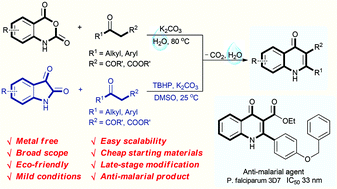
Eco-friendly decarboxylative cyclization in water: practical access to the anti-malarial 4-quinolones
Abstract
An environmentally benign decarboxylative cyclization in water has been developed to synthesize 4-quinolones from readily available isatoic anhydrides and 1,3-dicarbonyl compounds. Isatins are also compatible for the reaction to generate 4-quinolones in the presence of TBHP in DMSO. This protocol provides excellent yields under mild conditions for a broad scope of 4-quinolones, and has good functional group tolerance. Only un-harmful carbon dioxide and water are released in this procedure. Moreover, the newly synthesized products have also been selected for anti-malarial examination against the chloroquine drug-sensitive Plasmodium falciparum 3D7 strain. 3u is found to display excellent anti-malarial activity with an IC50 value of 33 nM.
Eco-friendly decarboxylative cyclization in water: practical access to the anti-malarial 4-quinolones
ethyl 2-(4-(benzyloxy)phenyl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (3u) White solid, m.p. 288-289 oC;
1H NMR (600 MHz, DMSO-d6) δ 12.14 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.72 (ddd, J = 8.4, 7.1, 1.5 Hz, 1H), 7.64 (d, J = 8.3 Hz, 1H), 7.52 (td, J = 8.5, 1.7 Hz, 1H), 7.43 – 7.35 (m, 4H), 7.29 – 7.21 (m, 4H), 7.10 (td, J = 7.5, 0.5 Hz, 1H), 5.17 (s, 2H), 3.91 (q, J = 7.1 Hz, 2H), 2.00 (s, 1H), 0.83 (t, J = 7.1 Hz, 3H) ppm;
13C NMR (150 MHz, DMSO-d6) δ 174.1, 166.2, 156.2, 148.0, 139.8, 137.2, 132.8, 132.0, 130.5, 129.4, 128.7, 128.2, 127.6, 125.5, 125.2, 124.3, 123.6, 120.9, 118.9, 116.4, 115.8, 113.5, 70.2, 60.2, 14.0 ppm;
HRMS (ESI) calcd for [C25H21NO4+H]+ 400.1471, found 400.1463.
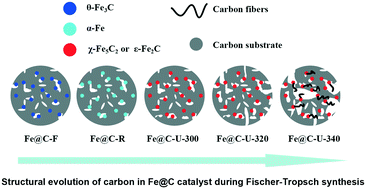
Structural evolution of carbon in an Fe@C catalyst during the Fischer–Tropsch synthesis reaction
Structural evolution of carbon in an Fe@C catalyst during the Fischer–Tropsch synthesis reaction
Abstract
A pseudo-in situ research method was applied to provide insight into the structural evolution of carbon in an Fe@C catalyst at different stages of the Fischer–Tropsch reaction. Five typical stages of the catalyst were selected for in-depth structural investigation; these were: the fresh catalyst, reduced catalyst, and catalyst in the stable conversion period, in an increased-conversion period and at the inactivation stage. The results indicated that the integral structure of Fe@C constantly changed in the Fischer–Tropsch reaction. Iron carbide transformed from the Fe phase that was easily oxidized under high temperature Fischer–Tropsch conditions, and the carbon framework was completely destroyed in the reaction process, leading to a drastic decrease in the specific surface area of the material. This destruction could have two opposing effects: on the one hand, the loss of carbon could re-expose the active sites that have been covered by carbon at a reaction temperature of 320 °C and favor the reaction; on the other hand, the deposition of carbon could block the active sites and lead to inactivation when the reaction temperature is over 340 °C.
////////Fischer–Tropsch